7th Grade Science Final Review (Part 1)
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Solids
Take up space, has mass, and has definite volume
Liquids
Take up space, has mass, takes the shape of its container, and has definite volume
Gas
Takes up space, has mass, takes the shape of its container, and fills container
Solid (spacing and motion of particles)
Has molecules close together and moves slightly
Liquid (spacing and motion of particles)
Has molecules spread apart and moving more
Gas (spacing and motion of particles)
Has molecules widely spread apart and moving fast
Evaporation
Liquid to gas
Freezing
Liquid to solid
Melting
Solid to a liquid
Condensation
Gas to liquid
Sublimation
Solid to gas
Deposition
Gas to solid

What is the melting temperature of the above substance?
5oC

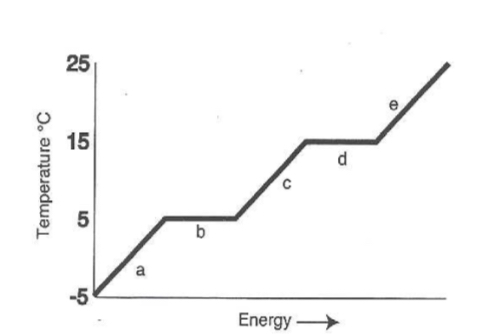
What is the freezing temperature of the above substance?
5oC


What is the boiling temperature of the above substance?
15oC


What is happening at “e”
A gas is being heated

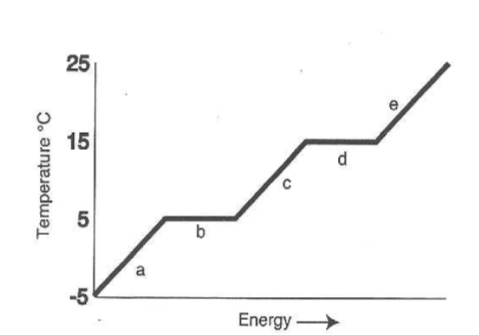
What is happening at “a”
A solid is being heated


What is happening at “b”
Melting of solid to liquid


What is happening at “c”
A liquid is heating up


What is happening at “d”
Boiling liquid to gas


In which section of the graph are atoms moving the slowest?
A


In which section of the graph is this substance in the liquid state?
C


Where is the least dense liquid?
Lamp Oil


Most dense liquid?
Karo Syrup Honey


How do you know Karo Syrup Honey is the most dense?
The higher the density, the more it will sink.


If we dropped an object in the column and it settled between the water and vegetable oil, what could you tell me about the object’s density?
The object is not denser than water, but denser than vegetable oil.

What is the definition of density?
The amount of mass in a given volume
How is density measured?
Mass divided by volume
Which state of matter has the greatest density of particles?
Solids